Rock Candy, and Experiment Extension for older kids
Have you ever looked at rock candy and wondered how it’s made? Rock candy is actually a collection of large sugar crystals that are “grown” from a sugar-water solution. Sugar, like many other materials, can come in many different physical states. As a solid it can either be amorphous, without shape, like when it forms cotton candy, or crystalline, with a highly ordered structure and shape, like when it forms rock candy crystals. Â
Crystals form when the smallest particles of a substance, the molecules, arrange themselves in an orderly and repetitive pattern. Molecules are too small for us to see moving around and arranging themselves, but you can get a rough idea of what this would look like by taking a small shallow tray and filling it with marbles, ball bearings, or other spheres. As you add more spheres, the bottom of the tray becomes covered, then the spheres must form layers on top of one another, and a structure or pattern emerges.
Â
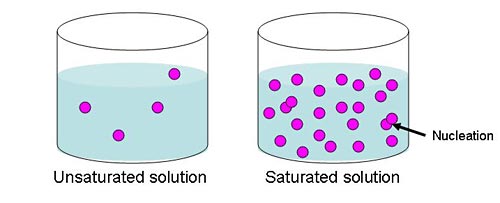
So how do the molecules of a substance get together to form a crystal? First there have to be enough molecules in one area that they have a high chance of bumping into one another. This happens when a solution, which is made up of a liquid and the compound that will be crystallized, is saturated. In the rock candy, the liquid is water and the compound is sugar. A solution is saturated when the liquid holds as much of the compound dissolved in it as possible. For example, when making rock candy, you dissolve as much sugar as possible in water to make a saturated solution. If you add more compound than can dissolve in the liquid, the undissolved bits remain as solids in the liquid. In a saturated solution, the molecules bump into one another frequently because there are so many of them. Occasionally when they bump into each other, the molecules end up sticking together; this is the beginning of the crystallization process and is called nucleation. Once several molecules are already stuck together, they actively attract other molecules to join them. This slow process is how the crystal “grows.”
Â
Â
Figure 1. This diagram illustrates the large number of molecules in a saturated solution. With so many molecules in the liquid, there is a high chance of them bumping into one another and creating a nucleation event.Â
Â
In this science fair project you will make a saturated solution of sugar and water in order to grow your own rock candy sugar crystals. You will compare the rate of growth between rock candy that is left to nucleate on its own in the solution, and rock candy that starts off with some assistance. To assist this rock candy, you will jump-start the nucleation process by adding sugar crystals, called seed crystals, to the string first.
Â
Here are some terms you should know, and questions you should think about before starting this science project. Have an adult help you look up these words and concepts.
Â
Amorphous solid
Crystalline solid (also known as crystal)
Molecule
Solution
Compound
Saturated
Nucleation
Seed crystal
Â
Questions
How do you make a saturated solution?
Which holds more sugar: cold water or hot water?
How do crystals grow?
What is nucleation?
Â
 Rock Candy
Rock Candy
Â
4 cups sugar    Â
2 cups water   Â
a small saucepan          Â
a wooden spoon          Â
a candy thermometer Â
a small, clean glass jar Â
a measuring cup           Â
cotton string   Â
a weight to hang on the string (such as a screw or galvanized washer)Â
waxed paper   Â
a pencil (to suspend the string in the jar)Â Â
Â
Heat the water in the saucepan over medium-high heat until it comes to a boil.           Completely dissolve the sugar in the boiling water, stirring continuously with the wooden spoon until the solution grows clear and it reaches a rolling boil.      Remove the solution from the heat, and then carefully pour it into the jar. Cover the jar with a small piece of waxed paper. Tie the weight to one end of the string, and then tie the other end to the middle of the pencil. The string should be about two-thirds as long as the jar is deep. Dip the string into the sugar solution, remove it, lay it on a piece of waxed paper, straighten it out, and let it dry for a few days. Gently suspend the prepared string in the solution and let sit at room temperature, undisturbed, for several days. You can check each day to see how much your crystals have grown. It’s tempting, but don’t touch the jar until the experiment is finished—it usually takes about seven days. At the end of the week, the crystals on your string should be clearly defined, with sharp right angles and smooth faces of various sizes. In the field of crystallography, these are called monoclinic crystals. Their shape is determined by the way the individual sugar molecules fit together, which is similar to the way the shape of a pile of oranges is determined by the shape of the individual oranges and the way they stack together.  Try adding food coloring or flavoring to your sugar syrup before making the rock candy.
Â
Â
Observations and Measurements
Â
Look at your jars once a day. What do you see? Are there any crystals growing? Where are the crystals? Which string has more crystals: the one that was or wasn’t seeded? Make a data table, like the one below, in your lab notebook and record your observations every day. While you actually prepared your strings on the “Day 1” step above, for the purposes of the experiment, Day 1 in your data table will be the day you made your solution and began running the experiment.
Â
|
Days Spent in Jar |
Observations |
|
Day 1 (the day the sugar-water solution was made) |
 |
|
Day 2 |
 |
|
Day 3 |
 |
|
Day 4 |
 |
|
Day 5 |
 |
|
Day 6 |
 |
|
Day 7 |
 |
Â
Make observations of your sugar-water solution jars for one week. On the seventh day, remove the strings from the jars and take measurements of your rock candy crystals.
Â
If there is a layer of hardened sugar syrup coating the top of your jar, you can use a spoon to gently break that layer before pulling out your sugar crystals.
Â
Briefly rinse the rock candy crystals in cold water, then leave them on a paper towel for 30 minutes to dry.
Â
Once you’ve recorded all your measurements and observations, you can enjoy all your hard labor by eating the rock candy you grew!